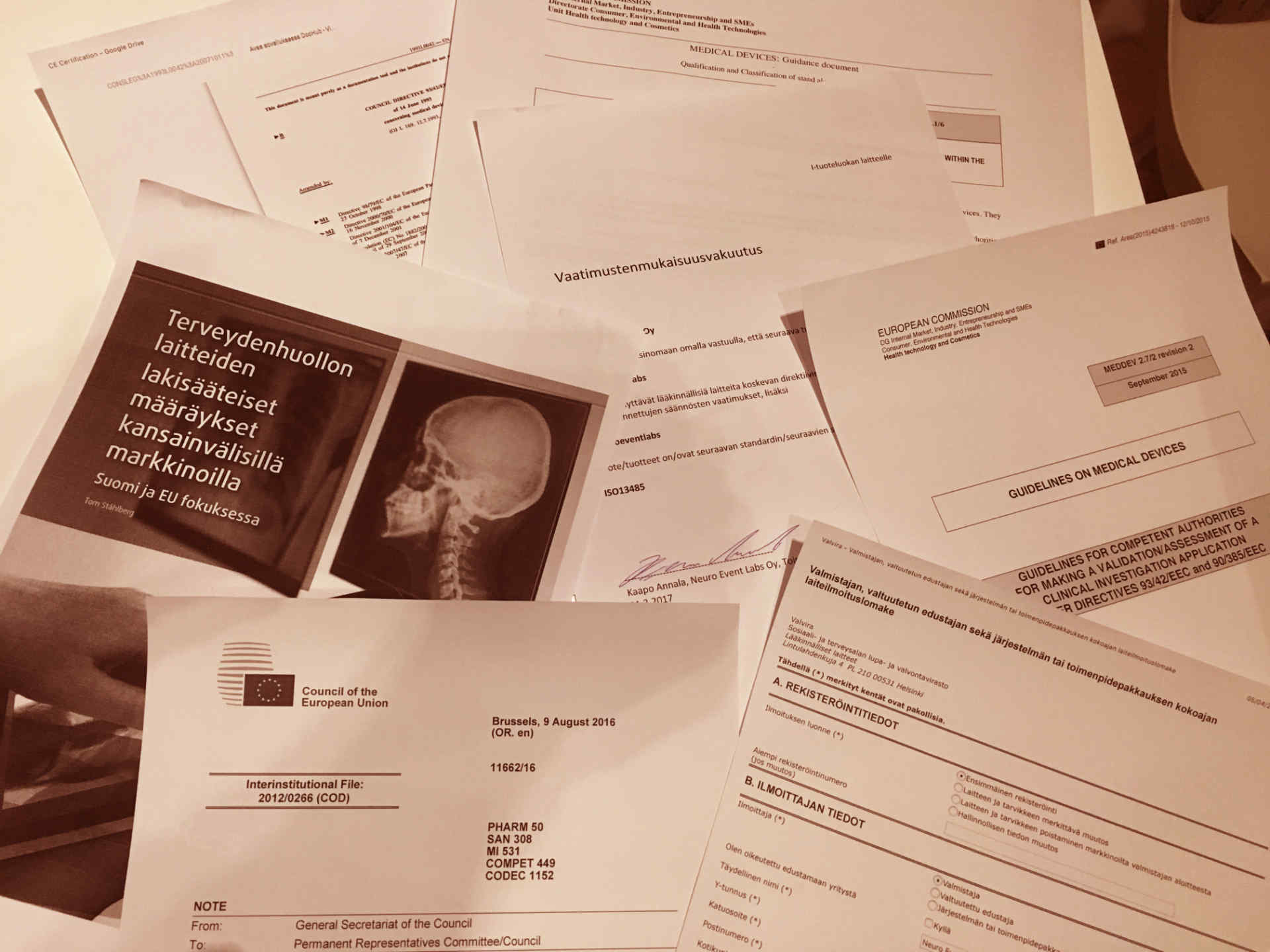
CE Marking of Medical Devices
The process for CE marking a medical device is a complicated landscape. In order to get an understanding of a process and find out what should be done, one encounters a significant amount of literature to process. Thankfully, there are people and services that can simplify this puzzle significantly. If you don’t want to read every page of every EU directive, their guidelines, hard-to-decipher pages of Finnish law, and make tens of phone calls just to understand how the actual process works, I can recommend some without hesitation. For Neuro Event Labs, CE marking is very important. We want to make sure that patient security and their data privacy meets the highest possible standards. That is where the CE marking process helps. To verify that everything possible has been taken into account, and that all possible risks and their mitigation has been recognized and thought beforehand, we implemented a quality management system which encompasses the relevant aspects of the applicable guidelines, standards and regulatory requirements.
For NEL, CE marking is not simply a one-time tick in the box, but continuous development following our product evolution. When new features and use cases are introduced in our product, we evaluate the impact of these changes for patient security and product quality. It is built into our engineering process. The CE mark is also a signal to patients and healthcare professionals that we take quality, availability, and privacy seriously.
While regulations primarily exist to prevent devices from having a negative impact on the patient’s safety and data confidentiality, they also force you to think and document product usage, user experience, performance, and technical solutions from the patient’s point of view. This is all good for us as well, as it helps us build a better, safer product.
Achieving the CE mark for Nelli® is an important milestone for us as the product enters clinical use (our focus for this year). We are well on track!


